UV-Visible Spectrophotometer is based on the idea that some of the light absorbed by molecules in a sample will be reflected. The amount of light absorbed depends on the light's wavelength and the molecule's structure. By measuring the absorbance of light at different wavelengths, we can determine the concentration of the absorbing species and identify unknown compounds.
UV-Vis spectroscopy: Definition
UV-Vis spectroscopy is a type of absorption spectroscopy that measures the amount of light absorbed by a sample as a function of wavelength. You can use it to determine the concentration of absorbing species in a sample and identify unknown compounds. This article will describe the principles of UV-Vis spectroscopy and provide some examples of its applications. As this spectroscopy technique is based on light, let's first explore the properties of light.
Light is a type of electromagnetic radiation consisting of electric and magnetic fields that oscillate at right angles to each other. The wavelength of light is the distance between two electric field peaks, and its frequency is the number of wave cycles that occur per second. The speed of light in a vacuum is always the same, but it slows down when traveling through a medium such as air or water.
The wavelength of light determines its color. Visible light is the portion of the electromagnetic spectrum that our eyes can see, and it has wavelengths in the range of 400-700 nm. Ultraviolet (UV) light has shorter wavelengths than visible light, while infrared (IR) light has longer wavelengths.
Light can be scattered, absorbed, or transmitted when it hits a material. Scattering occurs when the light is reflected in many directions, which makes objects visible. Transmission occurs when the light passes through the material without being scattered or absorbed.
Absorption is when the light is absorbed by the material, which is what UV-Vis spectrophotometer is based on. When an atom or molecule absorbs a photon (a particle of light), its energy is transferred to the electron of that atom or molecule. This raises the electron to a higher energy level, and it is this increase in energy that we measure in UV-Vis spectroscopy.
Working Process of a UV-Vis Spectrophotometer
A UV-Vis spectrophotometer consists of a light source, a wavelength selector, and a detector. The light source is usually a lamp that emits light over a wide range of wavelengths. The wavelength selector is used to select the desired wavelength of light, and the detector measures the amount of light absorbed by the sample.
Wavelength Selector
The wavelength selector is used to select the desired wavelength of light. The most common type of wavelength selector is the diffraction grating, which splits the light into its component wavelengths. The second type of wavelength selector is the monochromator, which uses a prism or diffraction grating to select a single wavelength of light. A monochromator separates light into its component wavelengths, and then a second diffraction grating or prism is used to select the desired wavelength.
The diffraction grating's groove frequency is the number of lines per millimeter, which determines the wavelength of light selected. The higher the groove frequency, the shorter the wavelength of selected light.
Absorption filters are commonly used in UV-Vis spectrophotometry, and they are used to remove unwanted wavelengths of light. The most common type of absorption filter is the interference filter, which consists of two thin layers of different materials. The thickness of the layers is carefully controlled so that only certain wavelengths of light are transmitted.
Interference filters are available with a wide range of wavelength cutoff values, which allows for selecting the desired wavelength of light. You can use these filters to eliminate light over a wide range of wavelengths, or they can be used to select a very specific wavelength of light.
Cutoff filters also allow the light of a certain wavelength to pass while blocking all other wavelengths. By combining short-pass and long-pass filters, bandpass filters enable a range of wavelengths to pass through.
Light Source
The light source is usually a lamp that emits light over a wide range of wavelengths. The mercury lamp is the most common type of UV-Vis spectroscopy, which emits light in the UV and visible regions of the spectrum. Mercury lamps are available with different emission lines, which allows for selecting the desired wavelength of light. Xenon lamps are also used in UV-Vis spectroscopy, providing a continuous light spectrum.
Tungsten and deuterium lamps are used for visible spectroscopy, while carbon arcs are used for UV spectroscopy. Lasers can also be used as light sources, and they can provide a very narrow range of wavelengths.
Detector
The detector measures the amount of light that is absorbed by the sample. The most common type of detector used in UV-Vis spectroscopy is the photodiode array (PDA) detector. This type of detector consists of an array of photosensitive diodes, each sensitive to a different wavelength of light. The output of the photodiode array is a plot of absorbance versus wavelength, called a spectrogram.
The PDA detector has the advantage of being able to take measurements at multiple wavelengths simultaneously. This allows for rapid measurement of absorption over a wide range of wavelengths. A photoelectric coating ejects electrons when exposed to light, and an electrode collects these electrons. The current that is produced is proportional to the intensity of the light.
Another type of detector you can use in UV-Vis spectroscopy is the photomultiplier tube (PMT). This type of detector consists of a photocathode that converts photons into electrons and a series of dynodes that amplify the electron signal. The output of the PMT detector is an electrical current proportional to the light's intensity.
Semiconductors can be used as detectors, called silicon photodiodes, in this capacity. Silicon photodiodes are used in UV-Vis spectroscopy because they have high sensitivity and linearity.
Parts of UV-Vis spectroscopy
The wavelength of light absorbed by a sample is called the absorption spectrum. The absorbance of a sample is measured at multiple wavelengths, and this data is used to create a plot of absorbance versus wavelength. The absorbance spectrum can be used to identify the substances in a sample.
The most common absorbance unit is the molar absorptivity coefficient, denoted by the symbol ε. The molar absorptivity coefficient measures how well a substance absorbs light at a particular wavelength. It is usually expressed in units of liters per mole-centimeters.
The absorbance of a sample is measured at multiple wavelengths, and this data is used to create a plot of absorbance versus wavelength. The absorbance spectrum can be used to identify the substances in a sample. Beer–Lambert's law is used to quantitatively analyze the absorbance spectra of samples. This law states that the absorbance of a sample is proportional to the concentration of the absorbing species.
The Beer-Lambert law is used to determine the concentrations of substances in solution. It is also used to determine the purity of substances. You can also use the absorbance spectrum to determine the identity of unknown substances.
The term optical density is sometimes used interchangeably with absorbance. However, the two terms have different meanings. Optical density measures how much light is absorbed by a sample, expressed in units of decibels per unit length.
Calibration curves are used in various applications, ranging from basic quality control to sophisticated analytics. A calibration curve may be constructed depending on the analysis's goal. For example, in UV-Vis spectroscopy, a calibration curve may be constructed using a set of known concentrations of the analyte. The absorbance of the unknown sample is then determined at the wavelength of interest, and this value is used to interpolate the concentration of the analyte in the unknown sample.
The wavelength at which the maximum absorption occurs is called the λmax in UV-Vis spectroscopy. The λmax can be used to identify unknown substances. Each substance has a characteristic λmax dependent on the molecule's structure. The molar extinction coefficient, denoted by the symbol ε, measures how well a substance absorbs light at a particular wavelength. It is usually expressed in units of liters per mole-centimeters. The molar extinction coefficient can be used to quantitatively analyze the absorbance spectra of samples.
For reliability and best practice, it is essential to use high-quality glassware and cuvettes when performing UV-Vis spectroscopy. Glassware should be free of cracks, chips, and other imperfections. Cuvettes should be made of high-quality materials such as quartz or borosilicate glass.
Advantages and Disadvantages of UV-Vis spectroscopy
UV-Vis spectroscopy is a versatile technique that has a wide range of applications. You can use it to identify and quantify substances in solution. You can also use it to determine the purity of substances. The main advantage of UV-Vis spectroscopy is its simplicity. It does not require complex apparatus and is relatively easy to learn. It's non‑destructive, meaning it can be used to analyze a sample without damaging it. You can measure quickly and accurately over a wide range of concentrations. The main limitation of UV-Vis spectroscopy is that it can only be used to measure solutions. It cannot be used to measure solid or gaseous samples. It is also inexpensive and widely available.
The main disadvantage of UV-Vis spectroscopy is that it can only be used to analyze substances that absorb light in the ultraviolet or visible region of the electromagnetic spectrum. Additionally, UV-Vis spectroscopy can only be used to measure the concentration of a substance if that substance has a known molar extinction coefficient. Some substances do not have a well-defined molar extinction coefficient, and these substances cannot be quantitatively analyzed using UV-Vis spectroscopy. Additionally, UV-Vis spectroscopy is sensitive to changes in temperature and pressure. Samples must be kept at a constant temperature and pressure to obtain accurate results.
Light scattering can also interfere with the accuracy of UV-Vis spectroscopy. Finally, UV-Vis spectroscopy is not always the best choice for quantitative analysis. For example, substances that absorb light in the near-infrared region of the electromagnetic spectrum can be more accurately analyzed using infrared spectroscopy.
Geometrical considerations are another important aspect to consider when using UV-Vis Spectrophotometer. The path length, or the distance that light travels through the sample, is an important factor in determining the absorbance of a sample. The path length is usually expressed in units of centimeters. In general, shorter path lengths are preferable, because they result in more accurate results.
Best UV-Vis spectrophotometers
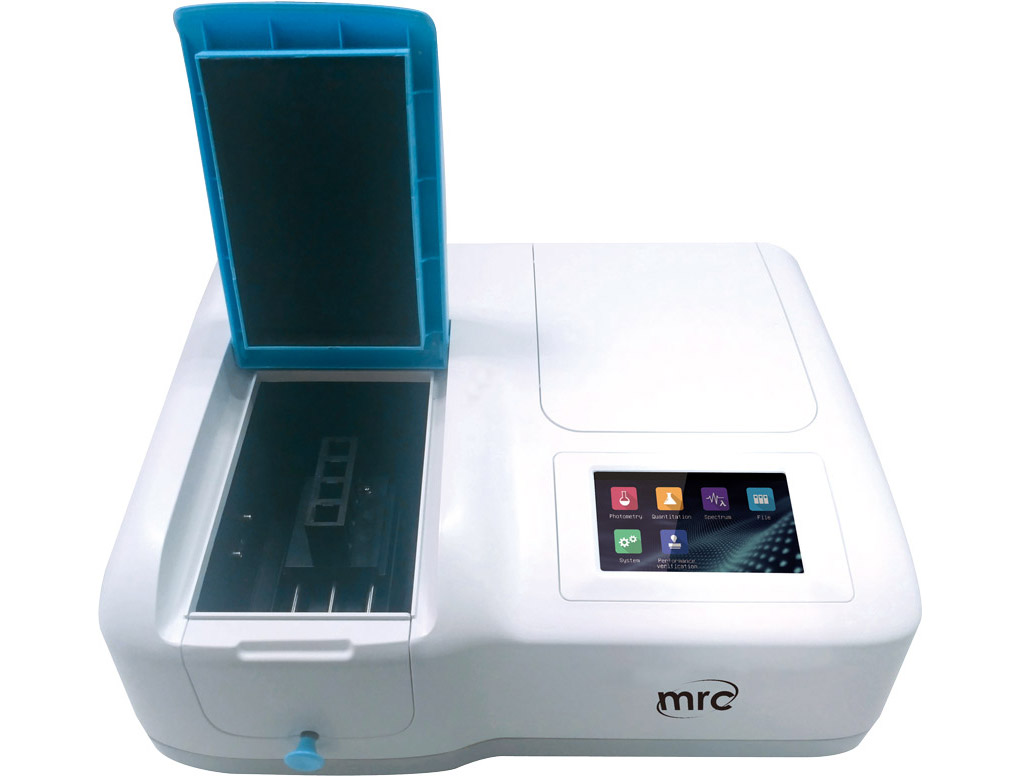
1.
Uv/vis Spectrophotometers series
Optical System: Single Beam.Scan speed: 4200nm/min.
Light Source: Tungsten Lamp, Deuterium Lamp.
Detector: Silicon Photodiode.
Display: 5 inches color screen (480x272).
Keypad: Resistive touch screen.
WAVELENGTH RANGE (NM):190-1010
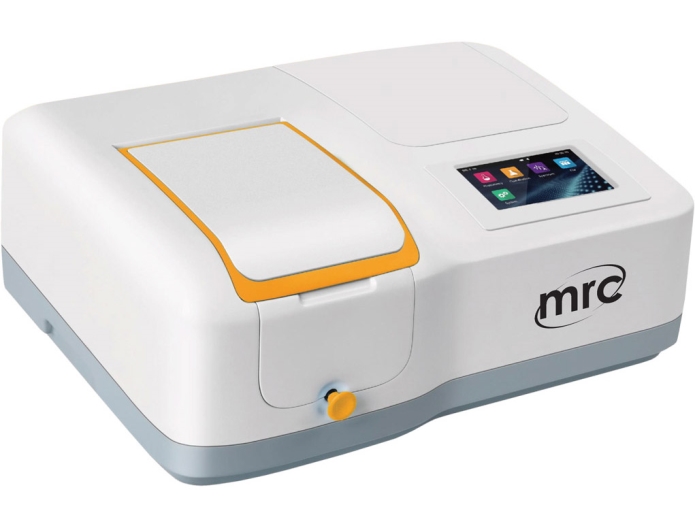
2.
UV/VIS SPECTROPHOTOMETER, XENON LAMP
Optical system: Split Double beam
Light source: Flash Xenon lamp
Spectral bandwidth: 2 nm
Wavelength range: 190~1100 nm
Spectrum scanning : Yes
Verity Uses of UV-Vis Spectroscopy
UV-Vis spectroscopy has a wide range of applications. It can be used to identify and quantify substances in solution and determine the purity of substances. Additionally, it can be used to study the properties of molecules, such as their structure, function, and interactions.
One common application of UV-Vis spectroscopy is in the field of food science. Food samples can determine the concentrations of vitamins, minerals, and other nutrients. Additionally, you can use it to identify and quantify food contaminants. Beverage analysis is another common application of UV-Vis spectroscopy. You can use it to determine the concentrations of dissolved solids, sugars, and other constituents in beverages. Additionally, it can be used to identify and quantify food additives.
UV-Vis spectroscopy is also commonly used in the field of medicine. It can determine the concentrations of drugs and other substances in blood and tissue samples. You can use it to monitor the progress of reactions in biological systems. For example, it can be used to study the effects of drugs on cells or to monitor the progress of chemical reactions in the body.
DNA and RNA analysis is another common application of UV-Vis spectroscopy. The absorbance of DNA and RNA can be used to determine their concentrations and study their structure and function.
Bacterial culture analysis is another common application of UV-Vis spectroscopy. It can be used to monitor the growth of bacteria in culture and determine the concentrations of antibiotics and other substances in bacterial culture media. OD measurements are also used in environmental science to determine the concentrations of pollutants in water and air samples.