A bomb calorimeter is a type of constant-volume calorimeter used in measuring the heat of combustion of a substance. It consists of a bomb or reaction container, which is surrounded by water in an insulated container. The bomb is filled with the substance to be tested and an oxygen gas, and then sealed. The bomb is heated using an electric current, causing the substance to combust and release heat. The heat released raises the temperature of the surrounding water, which can then be used to calculate the heat of combustion of the substance.
The calorimeter is a means of measuring the heat of chemical reactions or physical changes as well as heat capacity. A bomb calorimeter is a type of constant-volume calorimeter used in measuring the heat of combustion of a particular reaction.

Basically, a bomb calorimeter consists of a small cup to contain the sample, oxygen, a stainless steel bomb, water, a stirrer, a thermometer, the dewar or insulating container (to prevent heat flow from the calorimeter to the surroundings) and ignition circuit connected to the bomb.
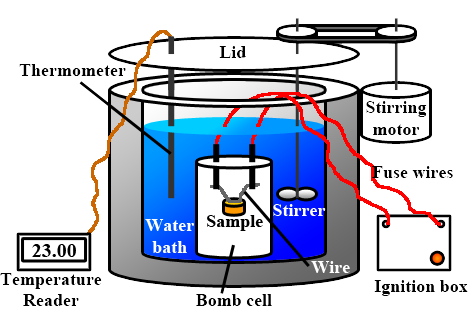
A bomb calorimeter is a type of constant-volume calorimeter used in measuring the heat of combustion of a substance. It consists of a bomb or reaction container, which is surrounded by water in an insulated container. The bomb is filled with the substance to be tested and an oxygen gas, and then sealed. The bomb is heated using an electric current, causing the substance to combust and release heat. The heat released raises the temperature of the surrounding water, which can then be used to calculate the heat of combustion of the substance.
Advantages
One of the main advantages of using a bomb calorimeter is its high precision and accuracy. The constant-volume design ensures that all heat released by the combustion reaction is absorbed by the water, allowing for accurate measurement of the heat of combustion. Additionally, the bomb calorimeter is able to measure a wide range of combustible materials, including liquids, solids, and gases.
When using a bomb calorimeter, it is important to ensure that the bomb is sealed properly to prevent any leakage of oxygen or the substance being tested. The water surrounding the bomb should also be at a known initial temperature, and the temperature change should be measured accurately using a thermometer.Types of bomb calorimeters
There are different types of bomb calorimeters, including adiabatic bomb calorimeter and isoperibolic bomb calorimeter.
Adiabatic bomb calorimeter is the most common type, it uses a bomb which is insulated to prevent heat loss, and the heat generated during the combustion reaction is transferred to the water surrounding the bomb, which causes the water to heat up.
Isoperibolic bomb calorimeter, it uses a bomb which is insulated to prevent heat loss, and the heat generated during the combustion reaction is transferred to the water surrounding the bomb, which causes the water to heat up, but in addition, the heat loss from the bomb is measured by the instrument, and it is used to calculate the heat of combustion.
A bomb calorimeter is a precise and accurate instrument used in measuring the heat of combustion of a substance. It is widely used in the fields of chemistry and engineering to determine the energy content of fuels, chemicals, and other materials.Bomb calorimeter in the food industry
A calorie is just a measurement of energy- the amount of energy needed to raise 1 gram of water 1 degree Celsius at standard atmospheric pressure. Calories in food are actually measured in kilocalories, so 1000 actual calories for every 1 Calorie listed.
Manufacturers measure calories using a bomb calorimeter. This process involved placing the food source in a sealed container filled with water, and burning the food with electrical energy. After the food had completely burned up, a measurement of the water temperature is done to see how many degrees it was raised and thus how many calories were used.
The calorimeters can be used to determine the calorific value of coal, coke, petroleum, cement black meal, solid biomass fuels and other combustibles.
What is a Bomb Calorimeter used for?
Bomb calorimeters are commonly used in:
- Food science to measure the energy content of foods.
- Fuel analysis to determine the energy output of various fuels.
- Material testing for research on the combustion properties of different materials.
MRC's Conformance with Standards:
- GB/T213-2008 Standard Test Method for Calorific Value of Coal
- ASTM D5865-2010 Test Method for Calorific Value of Coal and Coke
- ISO1928 Solid Mineral Fuels – Determination of gross calorific value by the bomb calorimetric method and calculation of net calorific value.