Spin coating is a widely used and versatile technique for depositing materials onto substrates with accurate and controllable film thicknesses For microscopic or spectroscopic investigations. Spin coating uses the centripetal force and surface tension of the solution to create an even film, making it quicker than other thin film coating methods. Spin coating is ideal for batch processing in both research and industrial settings.Spin coating is widely used in microfabrication of functional oxide layers on glass or single crystal substrates using sol-gel precursors, where it can be used to create uniform thin films with nanoscale thicknesses.
How spin coater works?
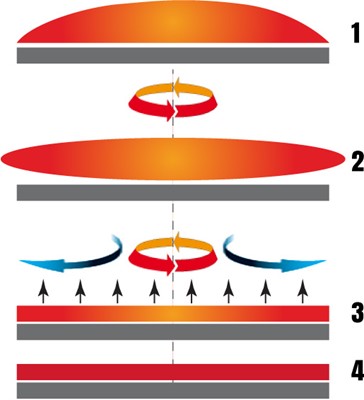
In spin coating the rotation continues as the liquid rotates from the ends of the substrate, until the desired film thickness is achieved. The solvent applied is usually volatile, and evaporates simultaneously.
MRC provide a variety of quality Spin Coater for use.All spin coaters are high quality products- View the catalog.

What is Spin Coating?
The method of spin coating is frequently used on surfaces to do thin coatings on. In this method, a uniform coating is formed after the combination of centripetal force and surface tension of the liquid whenever a solution of a substance and a solvent is spun at high speeds. Through spin coating, a thin layer, approximately a few nanometers, and a few microns thick, are produced after the leftover solvent has evaporated.
Spin coating is used extensively and has several uses. The process may be used to cover surfaces as tiny as a few millimeters squared up to flat panel displays with a diameter of one meter or more.
The main benefit of spin coating over other methods is its ability to make uniform coatings rapidly and easily. This technique is used in several businesses and technological domains.
The working principle in several steps:
- Deposition: The substrate, with the use of a pipette, is cast onto the solution. The centrifugal motion distributes the solution throughout the substrate despite the substrate being in dynamic or static spin coating.
- Spin up: After a spreading step at either a lower speed or quickly, the substrate achieves the appropriate rotation speed. The majority of the solution is now discharged from the substrate during this stage. The fluid becomes level once the drag equalizes rotational accelerations and leads to the speeds of rotation matching up.
- Spin-off: Now that viscous forces are dominant, the fluid starts to thin. Owing to interference effects, often, when the fluid is thrown off, the film's color shifts. The film is almost completely dried after the shifting of the color stops.
- Evaporation: After the fluid outflow ceases, solvent evaporation occurs. The volatility, vapor pressure, and environmental circumstances are a few factors on which the solvent evaporation rate depends.
Uses of Spin Coating:
It is used for coating surfaces with:
- Synthetic metals
- Nanomaterials
- Organic semiconductors
- Photoresistors
- Insulators
- Metal and metal oxide precursors
- Transparent conductive oxides
Several other substances are also used for spin coating.
Types of Spin Coating Techniques:
- Dynamic Dispense Spin Coating Technique: During the application procedure, a pipette is used to dispense a consistent quantity of coating using dynamic dispense spin coating. The object is rotated until the necessary rpm is achieved. Then the whole coating is applied as close to the center of the substrate as feasible. The speed and centrifugal force help to guarantee that the spin coating is consistent. With this procedure, the whole coating is applied simultaneously.
- Static Dispense Spin Coating Technique: In general, exceptional situations need that spin speeds be reduced below what a dynamic dispense spin coating method would require. Before commencing the spinning process, the whole coating substrate should be applied to the required material using this procedure. After the coating has been applied to the material, the spinning process begins, often at slower rates than with the dynamic dispense approach.
The Equation for Spin Coating Thickness:
The thickness of a film that has been spin-coated is proportional to the inverse of the squared spin speed as follows:
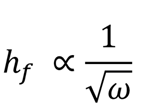
Here:
hf: final film thickness
ω: angular velocity/spin speed
Indicates: the thickness of a film will be reduced by one-fourth when it is spun at four times the normal speed.
Models:
To explain the ultimate film thickness from spin coating, many utilize a simple proportionality formula (stated as Equation 1) that is connected to spin speed. Having said that, the mentioned rule isn't always applicable, and in the absence of experimental data, it cannot be used to estimate the thickness of a film.This indicates that the assumption is made that stress will not change the viscosity of the utilized fluid.
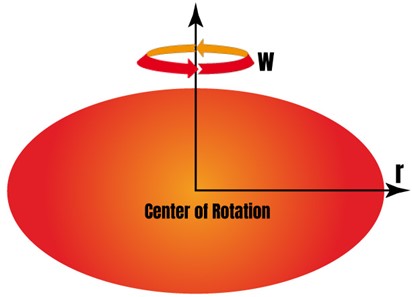
The behavior of a fluid that is non-volatile and viscous when it is placed on an infinitely spinning disc is described by the model:
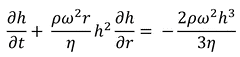
Here:
t: time since the start of the process
ω: angular velocity
r: the distance from the center of rotation
ρ: the density
η: the viscosity
h: the thickness of the fluid layer
∂h/∂t: the rate of change of thickness
∂h/∂r: the rate of spreading
The fluid film thickness may be described using Equation 3 if it is assumed that the film will be initially uniform.
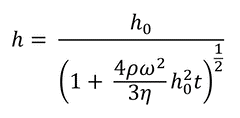
Here:
h0: the starting uniform thickness of the film
The precise thickness of the dry film that is produced in the end cannot be measured because this model does not take into account evaporation. It is possible to get an approximation of the dry thickness from the fluid film thickness by making use of the concentration of the solute and the solution density. A model that is more sophisticated is required in order to get an accurate result that takes into account the influence of both the viscosity of the solvent and the surface tension.
Meyerhofer Model:
In 1978, Meyerhofer made the first effort to add evaporation effects to his study. He updated the Equation 2 as follows:
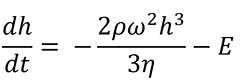
He included a solvent evaporation rate.
Here:
E: the uniform solvent evaporation rate
Meyerhofer hypothesised that:
- Early in the spin coating process, flow dominates the process of film thinning
- Later in the process, evaporation dominates the process.
Meyerhofer also proved that the film thickness might be determined analytically if the transition from fluid thinning to evaporation thinning is sudden. This can be done by assuming the film is thin enough to guarantee the solvent concentration stays uniform throughout the film's depth.
Clearly, this transition point will be the point at which flow-induced thinning is equivalent to evaporation-induced thinning, as shown here:
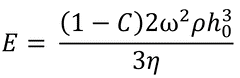
Here:
C: the volume fraction of solute in the film
h0: the film thickness between the two film-thinning regime's transition

This yields the ultimate thickness of the film as:
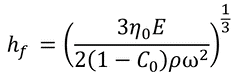
Here:
C0: the initial concentration of solute
η0 = η(C0)
Until the shift to evaporation-driven thinning commences, it is believed that the solute concentration will stay at C0. The thickness of the final spin-coated film may also be determined without the solvent evaporation rate. This may be achieved by making a number of assumptions, including that the airflow stays laminar throughout the operation.
This results in the total equation:
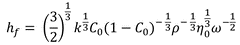
Here:
k: a constant specific to the coating solvent
k ≈ 1(-5) cm/s (-1/2): for typical spin coating solvents
The reader will then understand that this may be represented as:
hf (-1/2)
which is equal to Equation 1, demonstrating that the proportionality constant is a product of a number of other factors, such as solution density, viscosity, concentration, and solvent characteristics.
Contrary to Equation 7, the ultimate thickness of a spin-coated film is not necessarily proportional to (-1/2). This equation is based on a number of assumptions; if any of these assumptions are incorrect, the equation will fail.
The shift between both the thinning regimes not being sudden is an illustration of this. This had happened in rare instances when the spin coating process was interrupted prematurely during research. This indicates that the transition to evaporative thinning does not occur and that the connection between thickness and spin speed is distinct.
If a longer spin period had been used in these instances, it is likely that Equation 7 would have been valid. Systems may also differ from their predicted behavior if non-Newtonian fluids fail to transform into a Newtonian flow prior to becoming immobilized by evaporative thinning. This often happens at the gelation point or in colloidal fluids.
Using a "spin curve" is often the simplest method for establishing the connection for a specific solution. This may be accomplished by spin-coating a solution at different spin speeds and measuring the film thickness. Semi-empirical models consisting of Meyerhofer's equation and experimental data have also proven to be effective.
Benefits of spin coating:
- High uniformity is produced due to high spin rates, which facilitates drying periods.
- Compared to other technologies, spin coating is a fairly inexpensive approach to batch-process individual substrates.
- It eliminates the need for heat treatment post-deposition in many cases.
- It is useful for both research and quick prototyping because of the ease with which a procedure can be set up, as well as the thin and homogeneous coating that can be obtained at varying thicknesses.
Cons of spin coating:
- A lot of waste is caused because the actual material utilization in a spin coating process is often relatively low, with the remaining being discarded.
- As a single substrate process, the spin coating has comparatively low productivity compared to roll-to-roll techniques.
- Some nanotechnologies need time to self-assemble or crystallize, which might be hindered by the rapid drying periods.